Post Market Insights our solution to your Post Market Surveillance challenge
The Post-market Surveillance (PMS) Statutory instrument (SI) laid in Parliament this month is the first major update to the framework of medical device regulations in Great Britain, led by the Medicines and Healthcare products Regulatory Agency (MHRA).
In 2021, the MHRA consulted on the ‘Future Regulation of Medical Devices in the UK’ in response to recommendations set out in the Independent Medicines and Medical Devices Safety (IMMDS) review, published in 2020.
Responses to the consultation were strongly supportive of introducing clearer and more robust PMS requirements to improve patient and public safety and called for closer alignment with international approaches.
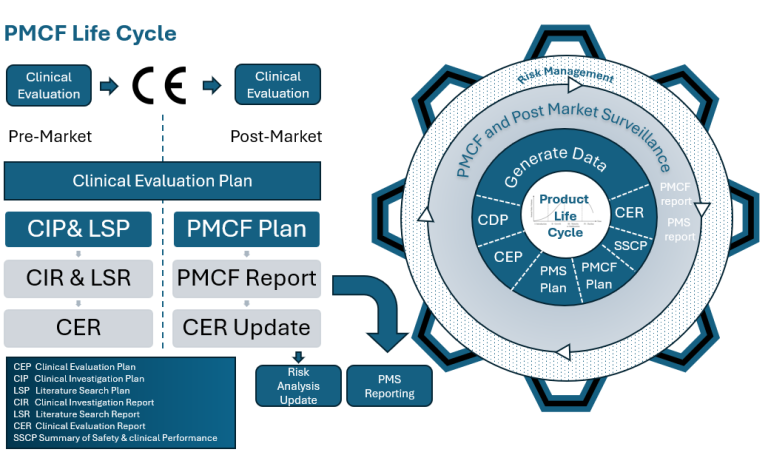
Post Market Clinical Feedback Life Cycle
Post-Market Surveillance Feedback for Wound Dressings
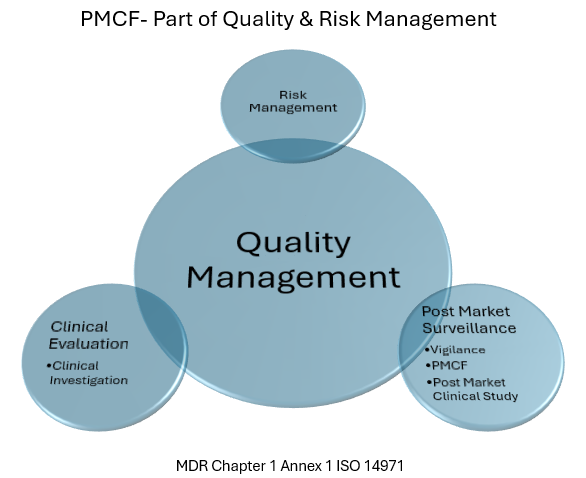
Under the new Medical Devices Regulation (MDR), specifically Annex XIV, Part B, manufacturers are now required to establish and maintain a Post-Market Surveillance (PMS) system for each medical device. This system must be continuously updated based on the device’s risk classification and type.
The MDR places increased importance on post-market surveillance, including proactive monitoring of device performance for recertification, annual safety updates for high-risk devices, and swift incident reporting.
A PSUR (Periodic Safety Update Report) is a key requirement under the European Medical Device Regulation (MDR 2017/745), aimed at ensuring the continued safety and performance of medical devices throughout their lifecycle. It is primarily focused on post-market surveillance and risk management.
What is a PSUR?
A PSUR is a document that manufacturers of medical devices are required to compile and submit regularly.
It provides an ongoing assessment of the benefit-risk profile of a device by gathering data from the field and post-market activities. The PSUR helps in identifying trends, emerging risks, and any necessary corrective actions for ensuring the safety and effectiveness of the medical device.

User Surveys
User surveys are a key part of the PMCF plan and under MDD equivalence data is not permitted.
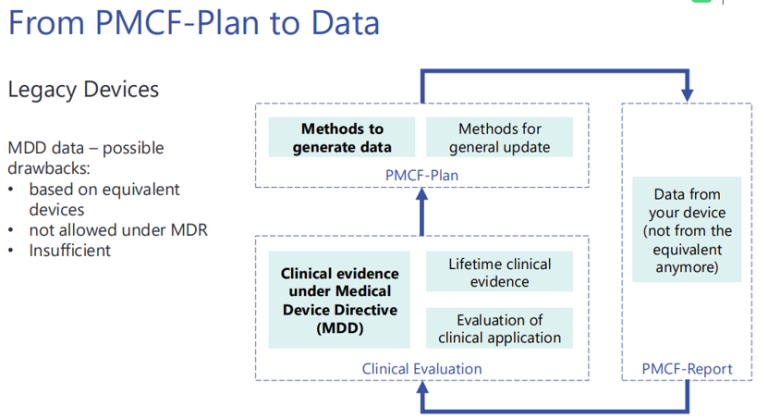
PMCF Plan
Your PMCF is only as good as the device specific data obtained from the market.
Frequency of PSUR Submission
- Class IIa and IIb devices: A PSUR must be updated at least every two years.
- Class III and implantable devices: The PSUR must be updated and submitted to the Notified Body annually.
Post Market Surveillance Feedback for Wound Dressings
The Challenge: Whilst in-house Regulatory & QA Departments are well versed in handling the constant stream of MDR hurdles facing them, one key problem still remains, collecting product relevant feedback from the professionals that use their devices.
Collecting clinical feedback in the wound care industry is notoriously challenging.
Complex Supply Chains
- Indirect Distribution Channels: Wound dressings are often sold through distributors, wholesalers, and various intermediaries before reaching the end user (clinicians or healthcare facilities).
- Fragmented Feedback Sources: Feedback can come from multiple, non-standardised sources, including hospitals, clinics, home care services, and even patients, complicating efforts to consolidate and analyse the data efficiently.
Limited Direct Clinician Engagement
- Lack of Direct Access to Clinicians: Regulatory teams often have limited direct engagement with the clinicians using the products, frequently the task is delegated to the not always co-operative sales team.
- Feedback Delays: Clinicians may only report issues when there is a significant problem, resulting in a lack of real-time feedback on product effectiveness or minor but recurring issues. Minor incidents may go unreported if they are seen as common or insignificant by clinicians, despite being critical for regulatory surveillance.
Regulatory Burden and Compliance Requirements
- High Volume of Data: Regulatory requirements such as the MDR necessitate continuous and comprehensive data collection. Keeping up with these requirements, particularly for high-risk devices, can be overwhelming for manufacturers.
- Data Integration: Compiling data from diverse sources into a format that meets the stringent requirements for post-market surveillance can be time-consuming and resource-intensive.
- Data Privacy Concerns: Navigating data protection regulations (e.g., GDPR) may also complicate the collection and sharing of patient-specific feedback.
Our Solution: To overcome these challenges, Med Dev Services has partnered with Wound Care People, a leading medical education provider in the wound care sector, to introduce Post Market Insights™. This comprehensive Post-Market Clinical Feedback (PMCF) service delivers brand specific post-market surveillance data, helping our clients stay compliant with MDR requirements.
Targeted feedback on your device, designed by auditors
Post Market Insights™
- Designed specifically to meet the regulatory demands of MDR audits
- Helps collect, analyse, and report clinical data for regulatory submissions
- Ensures manufacturers are audit-ready and compliant with PMS and PMCF requirements
Company Number 15734676
©Copyright. All rights reserved.
We need your consent to load the translations
We use a third-party service to translate the website content that may collect data about your activity. Please review the details in the privacy policy and accept the service to view the translations.

